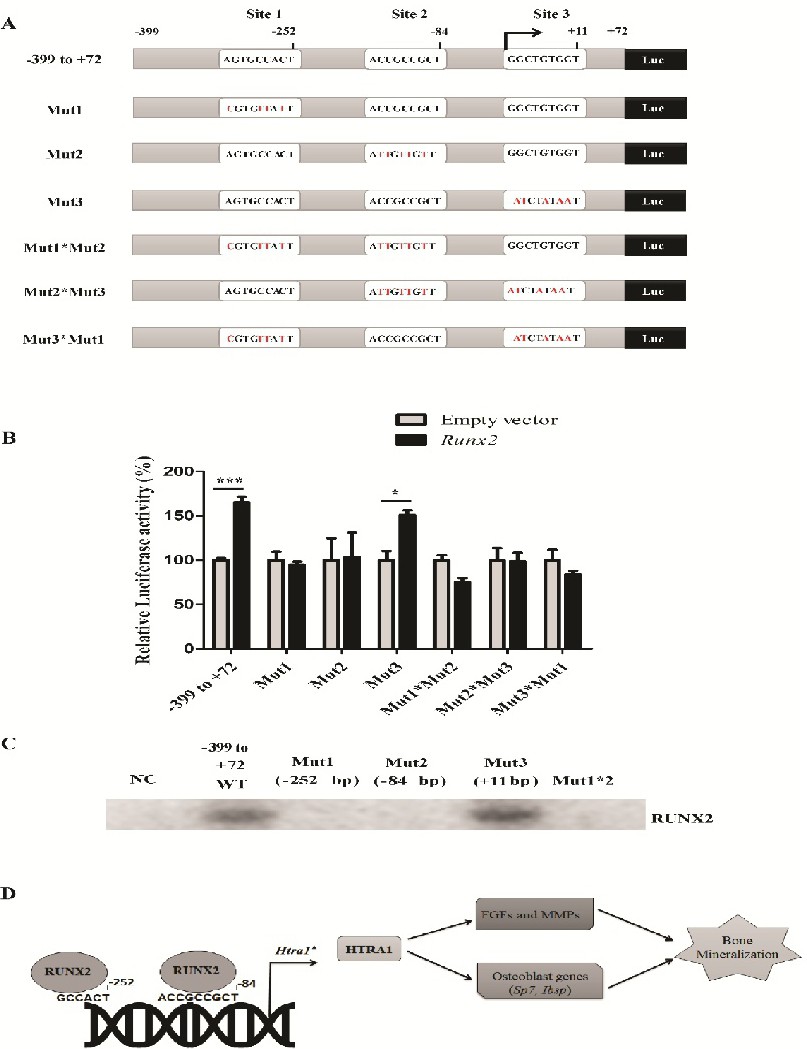
Fig. 7. Mutation of putative RUNX2 binding sites at -252 bp and -84 bp in the Htra1 proximal promoter abrogates the activation of Htra1 by Runx2. (A) Schematics diagram showing three putative RUNX2 binding sites on the 0.4 kb Htra1 promoter (-399 to +72 bp) and site-directed mutagenesis of putative binding sites at -252 bp (Mut1), -84 bp (Mut2) and +11 bp (Mut3) alone or in combination in the Htra1 promoter. (B) Relative luciferase activity of wild-type and mutated Htra1 promoter fragments (-399 to +72 bp) after overexpression of Runx2. Runx2 promotes the luciferase activity of the wild-type Htra1 promoter (-399 to +72 bp) and promoter construct with mutation of the site 3 at +11 bp (Mut3), whereas it failed to induce luciferase activity of the Htra1 promoter constructs with mutation of binding site 1 (Mut1) or site 2 (Mut2) alone or in combination. Relative luciferase activity was normalized to empty vector and represented as percentage activity. Mean ± S.E.M; two-way ANOVA with Bonferroni posthoc test; *, p<0.05; ***, p<0.001. Streptavidin-agarose pull-down assay (C) showing the interaction of RUNX2 with biotinylated wild-type Htra1 promoter probe (-399 to +72 bp) and mutated probes of site +11 bp (Mut3), however, RUNX2 failed to bind to mutated probes of sites -252 bp (Mut1) or -84 bp (Mut2). (D) Schematics showing the role of Htra1 in osteogenic differentiation. RUNX2 binds to two binding sites at -252 bp and -84 bp in the proximal Htra1 promoter to induce Htra1 mRNA expression in primary mesenchymal osteoprogenitor cells. Htra1 induces FGF pathway-related genes and Mmp's as well as osteoblast genes, such as Sp7 and Ibsp, to promote bone matrix mineralization.